Photoelectrochemical devices integrate the processes of light absorption, charge separation and catalysis and are therefore an interesting complement to currently existing life support systems for the realization of long-term space missions and surface habitats on Moon and Mars due to significant advantages of reduced weight and volume in comparison to traditionally employed electrochemical cells powered by PV.
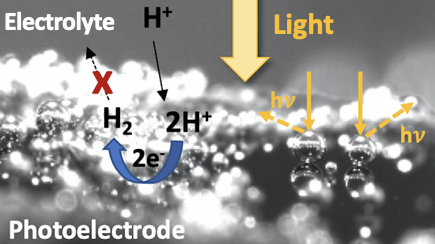
Other advantages are the high system tunability, the ease with which an electric field can be created at the semiconductor/liquid junction and the possibility to employ cost-efficient, earth-abundant electrocatalysts. The application of (photo-)electrochemical devices in reduced gravitation is however challenged due the absence of buoyancy which causes hindered gas bubble release from the electrode surface and mass transfer limitations of reactants and products to and from the electrode surface. In this joint project between the University of Warwick/UK and EPFL/Switzerland, we will investigate the impact of reduced gravitation on the performance of photoelectrochemical devices i.e., the oxygen evolution reaction (OER), the hydrogen evolution reaction (HER) and the CO2 reduction reaction.
Employing theoretical model systems using ideal semiconductor-electrocatalyst systems based on tandem- and triple junction cells possessing ideal solar-to-hydrogen (STH)/CO2-to-hydrocarbon efficiencies, we will simulate the impact of reduced gravitation in terms of hindered gas bubble detachment from the electrode surface, reduced light absorption of the semiconductor as well as increased ohmic resistances in proximity of the electrode surface and mass transfer limited/diffusion controlled electrode reactions.
Our gained insights in these model systems will guide the process design of efficient and stable photoanodes and -cathodes for OER, HER and CO2 reduction as well as optimal electrolyte compositions for application in terrestrial and reduced gravitational environments.